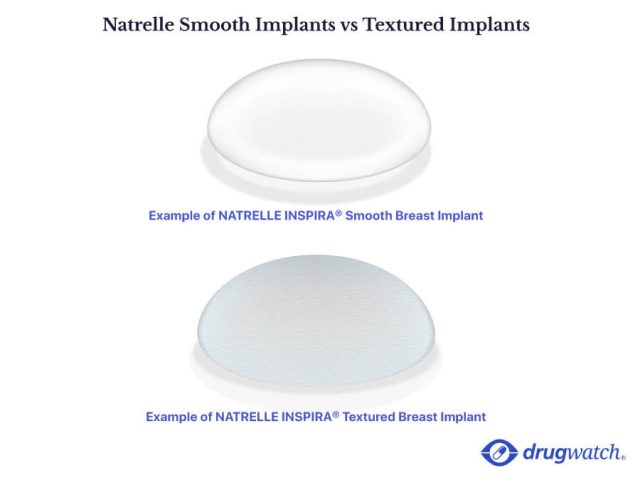
Textured Breast Implants Recalled For Cancer Risk

The communication announced that allergan was beginning a multi channel campaign to spread awareness about the biocell textured breast implant recall to patients who might not know they are at risk.
Textured breast implants recalled for cancer risk. Currently 38 other countries have recalled this implant because of its link to alcl. Following a request from the fda the company. The recall comes after a rare type of cancer called anaplastic large cell lymphoma alcl has been linked to textured breast implants. June 1 2020 the fda updated its safety communication about the allergan textured breast implant recall.
July 24 2019 pharmaceutical giant allergan has ordered the recall of all of its biocell textured breast implants after the fda flagged a series of reports that the implants were causing cancer. On june 1 2020 allergan launched a dedicated multi channel campaign to contact patients who may not be aware of the july 24 2019 recall of biocell textured breast implants and tissue. Allergan s textured breast implants will be recalled due to their link to a rare cancer the u s. The fda requested that allergan recall all biocell textured breast implants and tissue expanders marketed in the u s.